
编者按:9月13日,中国科学院上海药物研究所耿美玉、丁健和谭敏佳联合研究团队在Cell上发表了题为Targeting Epigenetic Crosstalk as a Therapeutic Strategy for EZH2-Aberrant Solid Tumors的研究成果,揭示了EZH2抑制剂对大部分实体瘤治疗无效的分子机制并提供了可能的协同抑制表观遗传交互调控解决方案,并对当前肿瘤的靶向治疗提出了示范性方案,对于当前EZH2抑制剂临床研究的困境破局具有重要的指导意义。这是中国药学史上第一篇发表在国际生物学权威杂志《细胞》(Cell)上的文章。

Cell论文共同通讯作者:耿美玉研究员(中)、丁健院士(左)谭敏佳研究员(右)
解读丨吴旭东 教授 (天津医科大学) 点评丨张 毅 教授 (哈佛大学)蒋 华良 院士 (中科院上海药物所)
肿瘤进展过程中遗传学变化和表观遗传学变化密切相关、相辅相承【1】。与遗传学变化不同的是,表观遗传学变化往往是可逆的,以染色质修饰相关蛋白作为治疗靶点,可使异常的表观基因组得以纠正。Polycomb家族基因EZH2在白血病和淋巴瘤病人中较多地发生活性增强的突变,在实体瘤中往往高表达,并且与肿瘤恶性程度和不良预后显著相关。目前,EZH2抑制剂在一部分血液系统肿瘤的治疗中表现不错,然而在实体瘤中,EZH2抑制剂仅仅对少数具有SWI/SNF成员基因突变或者BAP1突变的肿瘤表现出一定的效果【2】, 在胶质母细胞瘤中长期抑制EZH2的表达甚至会导致细胞激活多能性调控网络,增强细胞增殖和提高DNA损伤修复能力,反而促进肿瘤进展【3】。为了进一步扩大EZH2抑制剂在实体瘤的临床应用,有必要深入了解肿瘤细胞对EZH2抑制剂不敏感的分子机制。
9月13日,中国科学院上海药物研究所耿美玉、丁健和谭敏佳联合研究团队在Cell上发表了题为Targeting Epigenetic Crosstalk as a Therapeutic Strategy for EZH2-Aberrant Solid Tumors的研究成果,揭示了EZH2抑制剂对大部分实体瘤治疗无效的分子机制并提供了可能的协同抑制表观遗传交互调控解决方案,并对当前肿瘤的靶向治疗提出了示范性方案,对于当前EZH2抑制剂临床研究的困境破局具有重要的指导意义。

在这项研究中,研究人员系统地检测了83株EZH2高表达的肿瘤细胞对两种EZH2抑制剂(EPZ-6438和GSK126)的敏感性(IC50)。为了系统地鉴定与EZH2抑制剂敏感性相关的表观遗传标记,他们选取两株敏感细胞和4株不敏感细胞,通过基于SILAC的质谱分析比较了111种组蛋白标记在EZH2抑制剂处理前后的变化,发现所有细胞在EZH2抑制剂处理之后H3K27me2和H3K27me3的水平都显著下降,然而两类细胞中H3K27ac水平的变化表现出最大的差异:敏感细胞的H3K27ac水平几乎不变或者略有下调,而不敏感细胞的H3K27ac水平急剧升高。对43种代表性肿瘤细胞进行分析,他们发现H3K27ac水平增加的程度与细胞IC50显著正相关,说明EZH2抑制剂诱导激活的H3K27ac对细胞耐药起着重要作用。与此一致的是,敲低或者抑制负责催化H3K27ac的p300/CBP的确能使EZH2抑制剂不敏感的细胞变得敏感。然而,p300表达水平与敏感性无关,作者然后发现p300的募集蛋白MLL1在不敏感细胞中显著高表达,而敏感的淋巴瘤细胞几乎不表达MLL1;紧接着他们证实了MLL1敲低也能使EZH2抑制剂不敏感的细胞变得敏感,MLL1抑制剂的作用还有待确认。
为了系统探究H3K27me3逆转成为H3K27ac导致肿瘤细胞对EZH2抑制剂耐药的机制,作者对一株敏感细胞和两株不敏感细胞进行了H3K27ac ChIP-seq、RNA-seq和蛋白质组学分析,发现在不敏感细胞中EZH2被抑制之后H3K27ac水平的增加导致了多种致癌信号通路的激活,体内成瘤模型甚至显示某些肿瘤在EZH2抑制剂处理的情况下反而进展更快。为了干预H3K27ac水平升高导致的EZH2抑制剂耐药,作者尝试了联用BRD4抑制剂(下图),体外体内实验都证实BRD4抑制剂能恢复不敏感细胞对EZH2抑制剂的敏感性,抑癌效果显著增强。
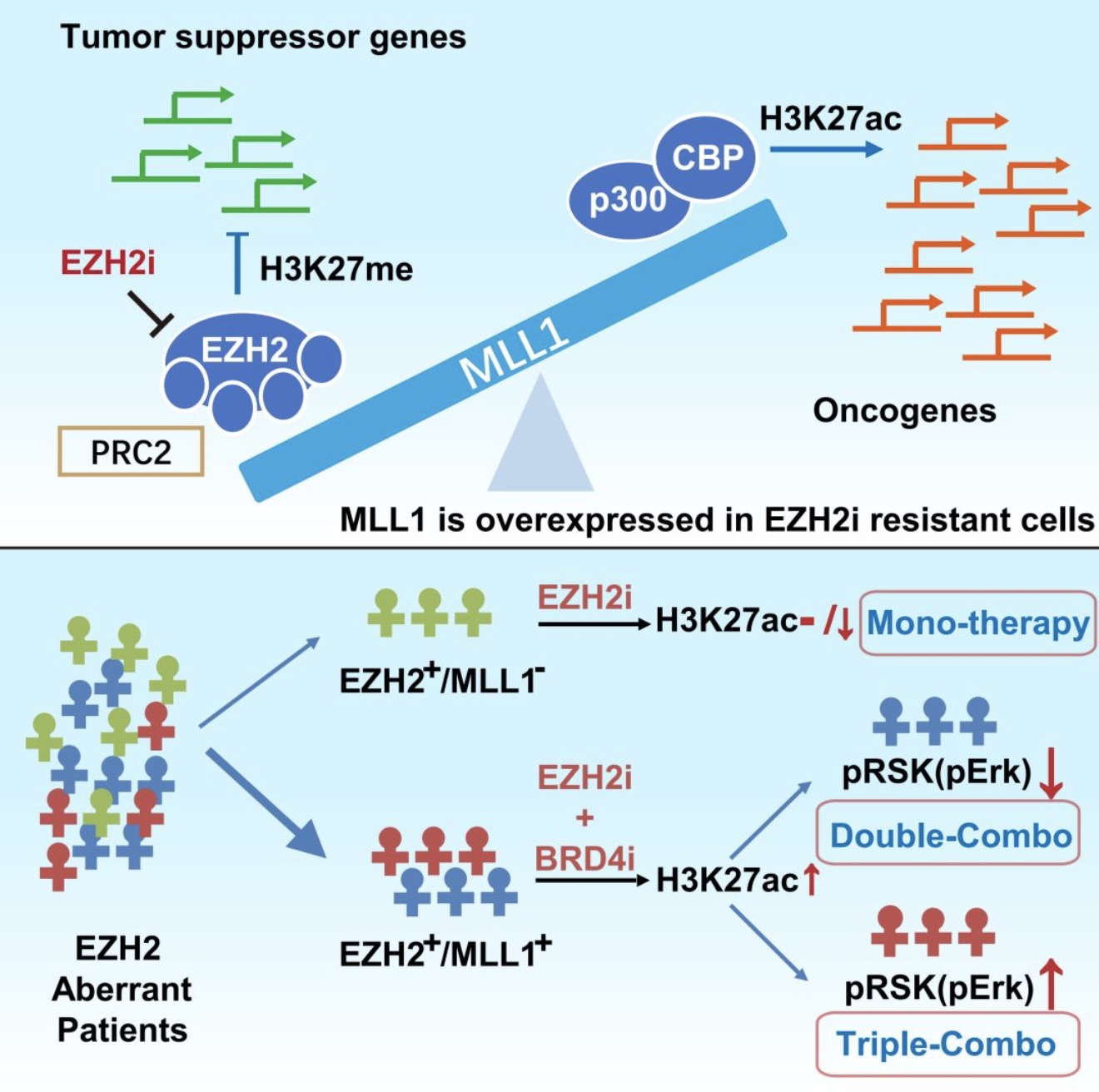
尽管如此,二联疗法在一部分肿瘤中的抑瘤作用仍然不够显著。质谱分析显示,二联疗法显著地激活了这些肿瘤细胞中的某些致瘤信号通路,比如MAPK通路。据此,作者在二联疗法基础上再加入ERK抑制剂,极强地抑制了肿瘤的生长,而且安全剂量情况下对动物的毒性较小。这种三联疗法阻断了多种致瘤途径,因此,尽管某些肿瘤细胞中EZH2可能同时发挥酶活性不依赖的作用【4】,该疗法可能仍然有效。此外,本文体内实验模型所用的是免疫缺陷小鼠,考虑到EZH2抑制剂也会提高杀伤性T细胞浸润【5】和NK细胞杀伤活性【6】,这一三联疗法在正常免疫环境下的抗肿瘤疗效依然很有希望。
因此,这项突破性工作不仅揭示了EZH2抑制剂对大部分实体瘤治疗无效的分子机制,同时提供了可能的解决方案,使EZH2抑制剂的临床应用范围得以扩大,而且也昭示着对表观遗传调控更深入细致的理解有望带来更特异、更有效的抗癌靶向治疗手段。
参考文献
1. Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153(1):38-55. 2. Helin K, Minucci S. The role of chromatin-associated proteins in cancer. Annu Rev Cancer Biol . 2017; 1: 355-77. 3. de Vries NA, Hulsman D, Akhtar W, de Jong J, Miles DC, Blom M, et al. Prolonged Ezh2 Depletion in Glioblastoma Causes a Robust Switch in Cell Fate Resulting in Tumor Progression. Cell Rep. 2015;10(3):383-97. 4. Kim K, Kim W, Howard T, Vazquez F, Tsherniak A, Wu J, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med. 2015; 21(12): 1491-96. 5. Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249-53. 6. Bugide S, Green MR, Wajapeyee N. Inhibition of Enhancer of zeste homolog 2 (EZH2) induces natural killer cell-mediated eradication of hepatocellular carcinoma cells. Proc Natl Acad Sci U S A. 2018;115(15):E3509-E18.
张毅(哈佛大学教授,HHMI研究员)
Polycomb group (PcG) proteins have long been known to be part of the cellular memory system involved in maintaining the spatial patterns of homeotic box (Hox) gene expression during embryonic development (Francis and Kingston, 2001). The Polycomb genes were originally named based on genetic screens in which gene mutations cause the generation of multi-sexcomb in flies. Due to his pioneering work in identifying genes (including PcG genes) controlling body patterning, Edward B. Lewis received the 1995 Nobel Prize in Physiology or Medicine (https://www.nobelprize.org/prizes/medicine/1995/lewis/facts/). Nevertheless, how PcG proteins maintaining the “off state” of Hox genes to control body patterning during embryonic development was not known till the discoveries that the two major PcG protein complexes PRC1 and PRC2 respectively possess histone H2A uniquitylation and histone H3 lysine 27 methylation (H3K27me3) activities to mediate Hox gene silencing (Cao et al., 2002; Wang et al., 2004). Around the same time, it was also found that the catalytic subunit of the PRC2, EZH2, is overexpressed in prostate cancer (Varambally et al., 2002). Subsequent studies indicated that overexpression of EZH2 is generally associated with various cancers (Comet et al., 2016) raising the possibility that EZH2 might be a promising target for cancer treatment. The biotech company Epizyme and the pharmaceutical company GSK were the first to develop small chemical inhibitors specifically targeting EZH2 with efficacy for hematological cancers (Knutson et al., 2012; McCabe et al., 2012). Despite the promising clinic results of these inhibitors in hematological cancers, whether these inhibitors are effective for solid tumors overexpressing EZH2 remains to be determined.
In this study, Huang et al. tested the EZH2 inhibitors (EZH2is) in a panel of 83 cancer cell lines that include both hematological and solid tumors and found that hematological tumor cell lines are relatively more sensitive to the inhibitors compared to the solid tumor cell lines. To determine why solid tumor cell lines are less sensitive to EZH2i, the authors analyzed 111 histone marks of EZH2i sensitive and insensitive cells and observed that the insensitive cells have a much higher H3K27 acetylation (H3K27ac) levels compared to those of the sensitive cells. Interestingly, the EZH2i sensitivity and drug-induced change in H3K27ac exhibited a reciprocal relationship suggesting that increased H3K27ac might contribute to cell’s resistance to EZH2 inhibition. Consistent with this notion, the authors found that the EZH2i insensitive cell lines express multiple oncogenes at a higher level which correlates with high levels of H3K27ac. Given previous studies demonstrating that H3K27ac is “write” by p300/CBP and “read” by the Bromodomain protein BRD4, the authors used a BRD4 inhibitor to block the H3K27ac function so that the EZH2i insensitive cells can be sensitized. Indeed, this combinational treatment significantly improved the efficacy of EZH2i in both cell culture and mouse xenograft models, supporting the author’s hypothesis as well as extending EZH2i’s therapeutic potential. Despite the use of BRD4 inhibitor improved the efficacy of EZH2i, a subset of cancer cells still escape the combinational treatment. To find out why this combined approach does not work for these cancer cells, the authors performed comparative ChIP-seq, RNA-seq, and proteomic analysis which identified a few oncogenic pathways, including the MAPK pathway, that escape the combined treatment. This observation prompted the authors to interfere the oncogenic MAPK pathway to further expand the therapeutic potential to a number of tumor models, including pancreatic and lung cancers.
In summary, using a comprehensive approaches, the authors demonstrate the existence of a complex network of cross talks of epigenetic modifications that regulate cancer cells’ response to EZH2i treatment. In addition, the study provides a strategy for patient stratification and therapeutic intervention. Specifically, for EZH2+/MLL- patients, EZH2i alone might be effective. However, for EZH2+/MLL+ patients, a combined EZH2i and BRD4i regiment might be needed. If this is still not effective, a triple combination that include the MAPK inhibitor might be considered. Given the generally encouraging xenograft results, clinic trial might be expected. Thus, this study provides a strong scientific bases for expanding the efficacy of EZH2i through combinational therapies.
References
Cao, R., Wang, L., Wang, H., Xia, L., Erdjument-Bromage, H., Tempst, P., Jones, R.S., and Zhang, Y. (2002). Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039-1043.
Comet, I., Riising, E.M., Leblanc, B., and Helin, K. (2016). Maintaining cell identity: PRC2-mediated regulation of transcription and cancer. Nat Rev Cancer 16, 803-810.
Francis, N.J., and Kingston, R.E. (2001). Mechanisms of transcriptional memory. Nature reviews Molecular cell biology 2, 409-421.
Knutson, S.K., Wigle, T.J., Warholic, N.M., Sneeringer, C.J., Allain, C.J., Klaus, C.R., Sacks, J.D., Raimondi, A., Majer, C.R., Song, J., et al. (2012). A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nature chemical biology 8, 890-896.
McCabe, M.T., Ott, H.M., Ganji, G., Korenchuk, S., Thompson, C., Van Aller, G.S., Liu, Y., Graves, A.P., Della Pietra, A., 3rd, Diaz, E., et al. (2012). EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492, 108-112.
Varambally, S., Dhanasekaran, S.M., Zhou, M., Barrette, T.R., Kumar-Sinha, C., Sanda, M.G., Ghosh, D., Pienta, K.J., Sewalt, R.G., Otte, A.P., et al. (2002). The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419.
Wang, H., Wang, L., Erdjument-Bromage, H., Vidal, M., Tempst, P., Jones, R.S., and Zhang, Y. (2004). Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873-878.
蒋华良(中国科学院院士、中科院上海药物所所长)
表观遗传领域是肿瘤治疗研究的前沿阵地,特别组蛋白甲基转移酶EZH2,因其在多种血液系统肿瘤和实体瘤中广泛存在基因扩增、激活突变或过表达,并与肿瘤发生发展密切相关,一直是肿瘤学基础研究及各大药厂新药研发追逐的热点(Kim and Roberts, 2016)。然而,在肿瘤学研究及抑制剂发现齐头并进的同时,对于EZH2抑制剂的疗效响应机制和敏感群体的认识却大大滞后,成为EZH2抑制剂临床转化的瓶颈。2018年8月,当前临床研究进展最快的EZH2抑制剂Tazemetostat(Epizyme公司研发)单药治疗EZH2突变的弥漫性大B细胞淋巴瘤(Diffuse large B cell lymphoma)宣布失败(NCT01897571),更加凸显深入系统认识EZH2抑制剂临床治疗背后的生物学机制的重要性与紧迫性。
中科院上海药物研究所耿美玉研究员、丁健院士和谭敏佳研究员的团队长期从事抗肿瘤药物敏感群和耐药机制研究。团队研究人员通过梳理以往关于敏感群体的研究发现,肿瘤中EZH2自身突变、EZH2功能相对的表观遗传调控复合物功能(或其底物的)异常,如SWI-SNF成员失活突变(Kim et al., 2015)和H3K27突变(Mohammad et al., 2017)会导致该群体对EZH2抑制敏感。已有认识将上述群体中EZH2抑制剂的治疗效应发挥归结于其底物组蛋白H3的27位赖氨酸甲基化(H3K27me)水平状态的变化。但是,一个显而易见的矛盾点是,EZH2抑制剂能普遍降低所有肿瘤的H3K27me水平,而治疗响应迥异。
对近百株肿瘤细胞EZH2抑制剂的敏感性和表观遗传动态修饰变化进行系统筛查,结合组蛋白修饰全景的检测分析方法,团队研究人员发现EZH2抑制剂可改变数十种组蛋白修饰状态、重塑肿瘤细胞内蛋白修饰格局。尤为值得关注的是,EZH2被抑制后,可导致H3K27位甲基化与乙酰化(H3K27ac)修饰的转换,且恰是这种修饰模式的转换决定了EZH2抑制剂治疗的不敏感。有关抑制EZH2可介导H3K27ac上调这一现象早在2010年就被报道(Pasini et al., 2010),但是,由于该研究及后继相关研究主要将该现象归结为蛋白修饰占位效应的体现,属继发效应,故并未引起领域的重视。研究团队通过组蛋白修饰谱系统分析揭示,H3K27me与H3K27ac的转换具显著有特异性;且在EZH2抑制剂引发的数十种组蛋白修饰改变中,只有H3K27ac变化与药物敏感性相关。H3K27me与H3K27ac作为基因转录开关的两个重要标志,其在转录调调控中的对立关系深为领域所知。这一关联性的存在提示,EZH2抑制剂在引发基因转录关闭的同时,还通过影响H3K27ac控制转录开启,具有双重角色。而H3K27ac开启的癌基因转录,是造成EZH2抑制剂不敏感的核心机制。
接下来的问题是,为什么EZH2抑制剂引起的H3K27me与H3K27ac转换主要在大部分的实体瘤中发生?团队研究人员发现,TrxG(Trithorax group)家族成员MLL1的表达水平是核心制约因素。在大多数实体瘤中,EZH2和MLL1均高表达,抑制EZH2的同时,MLL1招募p300和CBP形成复合物并催化H3K27ac修饰上调。反之,在MLL1低表达细胞中,抑制EZH2导致 H3K27me下调,却难以触发H3K27ac发生。这一发现提出了基于MLL1的H3K27me与H3K27ac的相互牵制决定EZH2抑制剂生物学效应的工作模型,揭开了PRC2和TrxG两大转录开关家族之间高度依存关系的新篇章。因此,EZH2抑制剂、MLL抑制剂以及p300和BRD4抑制剂,这些原本看似功能相悖的抑制剂,以MLL1募集功能为纽带,有了联合使用的可能。进一步研究证实EZH2和BRD4抑制剂联合用药在大多数EZH2高表达的肿瘤中疗效显著,为上述机制模式提供了有力的支持。
有意思的是,研究团队通过组蛋白修饰谱-转录谱-蛋白质谱-磷酸化谱的协同差异化分析发现,在一小部分肿瘤中,MAPK通路的异常激活是导致表观遗传修饰协同抑制仍然不能完全阻止肿瘤生长的重要因素。在此基础上,针对性联合抑制MAPK通路,可实现对肝癌、胰腺癌等难治性肿瘤的分群体组合治疗,效果显著。上述发现证实,协同抑制表观遗传交互调控的方案,尽管可实现对肿瘤转录异常的控制,但有些肿瘤依旧存留或被反馈激活的激酶信号通路,仍然可能是肿瘤细胞生存的驱动力量,这可能是表观遗传抑制剂获得性耐药产生的又一不可忽视的因素。
总之,该项研究着眼全局,全景分析了在广泛存在EZH2异常的肿瘤中,EZH2抑制剂如何诱发组蛋白修饰相互转变,如何与激酶信号通路的交互影响,并据此实现了对EZH2高表达肿瘤的精准治疗分层,并针对性提出了递进式个性化治疗方案,对于当前EZH2抑制剂临床研究的困境破局具有重要的指导意义,同时对于其他表观遗传类靶点药物敏感群体选择和耐药机制发现及联合用药策略制定,也均具有重要的示范作用。
参考文献
Kim, K.H., Kim, W., Howard, T.P., Vazquez, F., Tsherniak, A., Wu, J.N., Wang, W., Haswell, J.R., Walensky, L.D., Hahn, W.C., et al. (2015). SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nature medicine 21, 1491-1496.
Kim, K.H., and Roberts, C.W. (2016). Targeting EZH2 in cancer. Nature medicine 22, 128-134.
Mohammad, F., Weissmann, S., Leblanc, B., Pandey, D.P., Hojfeldt, J.W., Comet, I., Zheng, C., Johansen, J.V., Rapin, N., Porse, B.T., et al. (2017). EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nature medicine 23, 483-492.
Pasini, D., Malatesta, M., Jung, H.R., Walfridsson, J., Willer, A., Olsson, L., Skotte, J., Wutz, A., Porse, B., Jensen, O.N., et al. (2010). Characterization of an antagonistic switch between histone H3 lysine 27 methylation and acetylation in the transcriptional regulation of Polycomb group target genes. Nucleic acids research 38, 4958-4969.


